Breast Implant Rupture and High-Definition Ultrasound
It is not common among plastic surgeons to use ultrasound technology to examine the age and health of breast implants; however, at Aesthetic Plastic Surgical Institute, Dr. Daniel C. Mills has done so for over 10 years, long before this method was popularized. Dr. Mills can use ultrasound imaging to examine the integrity of implants and determine whether or not they need replacement. In addition, ultrasound imaging is complimentary for patients who had their implants placed by Dr. Mills.
With that in mind, note that breast implants typically last about 10 years. This is the length of time that Dr. Mills regularly cites for patients who are considering breast augmentation, and it is also the most common length of time covered under warranty by many breast implant manufacturers.
The fact is that, while many advances in implant design and the materials that compose breast implants have been made over the years, they are not impenetrable, and no matter how durable and state-of-the-art they may be, breast implants are subject to a certain amount of “wear” over time. If the internal scar tissue (capsule) constricts it will create a fold in the shell of the implant and, with daily activities, the implant shell will be subject to weakness. In some breast implant exchange procedures, Dr. Mills has seen “silent” ruptures—instances where the rupture is not noticeable—as early as six years after the implants were initially placed.
Dr. Mills does not recommend leaving a broken implant in place, regardless of whether it is actively causing problems. He firmly believes in the old axiom: “An ounce of prevention is worth a ton of cure.” Patients should have the integrity of their implants examined prior to their implant warranty expiring. Frequently, Dr. Mills tells his patients that it may be a good idea to do a breast implant exchange right before it’s been 10 years in case one has ruptured. That way, patients may get some relief financially from the manufacturer if the implant is both ruptured and under warranty.
The sections below provide more information on breast implant rupture and an innovative ultrasound technology available at our practice that can quickly identify ruptures and breaks in breast implants.
- What Are the Symptoms of a Ruptured Implant?
- Is Pain a Sign of Implant Rupture?
- Does a Ruptured Implant Cause Breast Implant Illness?
- Will Ruptures Show Up On Mammograms?
- How Can I Find Out If My Breast Implants Have Ruptured?
- What Is High-Definition Ultrasound?
- FDA Guidance on Breast Implant Labeling
What Are the Symptoms of a Ruptured Implant?
Whereas a saline rupture is often easy to detect, silicone ruptures are typically less noticeable without extensive testing. For instance, a saline rupture will generally be apparent when the implant flattens as the saltwater solution inside leaks into the body. The saline should be reabsorbed by the body and should not pose any danger to the patient, but the silicone shell of the implant usually requires surgical removal.
Meanwhile, silicone ruptures are often more complicated. In some cases, the rupture may not be outwardly noticeable at first – a phenomenon sometimes called a “silent rupture.” Alternatively, the implant rupture may leak silicone into lymph nodes, and/or produce granulomas of silicone around the implant. Other signs of a silicone implant rupture can include soreness, swelling, lumps, or inflammation.*
In Dr. Mills’ experience, the most common problem caused by a ruptured implant is capsular contracture (excessive scar tissue formation around the implant). In fact, Dr. Mills has found that the most common reason for late development of capsular contracture is a broken implant.
Is Pain a Sign of Implant Rupture?
Dr. Mills has found that pain is not necessarily an indication that an implant has ruptured unless capsular contracture is involved. Capsular contracture can cause pain; however, Dr. Mills estimates that, in about 75% of his patients with breast implants who say they are experiencing pain, it can often be attributed to the effect of a compound called “xanthene.” Xanthenes are the breakdown products of caffeine, which are stored in the ducts of the breasts. It is important to know that the breast is essentially a modified sweat gland, and if the ducts fill with excess xanthene byproducts, it can cause pain.
Dr. Mills has found that another common reason why women may experience pain in the breast is a change in her workout routine. There’s no question that, when an implant is placed below the chest muscle, the pectoral muscle and musculoskeletal system become slightly weakened, making it easier for individuals to suffer a pulled tendon or ligament, similar to a hamstring injury. Dr. Mills typically advises patients with breast implants to avoid working out their pectoral muscles after they have the implants placed subpectorally. For those who desire larger pectoral muscles, Dr. Mills may suggest larger breast implants. Ultimately, he believes that working out the chest can deform the aesthetic appearance of the breasts and increase the chance for capsular contracture.
Does a Ruptured Implant Cause Breast Implant Illness?
Breast implant illness (also called “BII”)* is a rare condition that has not officially been linked to breast implants, but is sometimes informally referred to as a side effect. Usually presenting as fatigue, “brain fog,” joint pain, headaches, or skin rashes among other symptoms, the condition is considered by some to be an autoimmune response to a foreign object inserted into the body (i.e., the breast implant).
Not only does implant removal have yet to be proven to stop the alleged symptoms of BII, but the issue is not thought to be related to implant rupture. If you are concerned about developing BII, rest assured that the condition is extremely uncommon. Dr. Mills will gladly provide you with more information about BII and other potential complications.
Will Ruptures Show Up On Mammograms?
In many cases, mammograms are able to detect whether breast implants have ruptured. A mammogram employs low-energy X-Ray technology to examine the health of the breasts, most frequently scanning them for breast cancer. While implant ruptures can also be detected on a mammogram, they cannot always be seen, which is why other techniques – such as ultrasound technology and MRI – are usually regarded as more effective in identifying ruptures.
Regardless, Dr. Mills encourages each patient to attend yearly mammograms to potentially catch any serious health risks before they develop. Because the presence of breast implants can make it more difficult for radiologists to detect cancer or other concerns, you are advised to let your mammogram technician know ahead of time that you have implants. This should give them the opportunity to plan an alternative course of action for your test.
How Can I Find Out If My Breast Implants Have Ruptured?
Other than physical exams and just listening to your body, there are a few select methods for detecting an implant rupture. MRI is widely regarded as one of the best tests to diagnose implant rupture; however, these advanced imaging tests are often expensive, costing approximately $1,500. If patients have access to a primary care physician, the physician can write them a referral for an MRI, for which insurance policies may provide benefits. Unfortunately, many women do not have that option, or they have an exclusion on their insurance that will preclude them from being able to get coverage for an MRI.
Fortunately, our practice offers another way to search for breast implant ruptures that has shown to be very effective. High-definition ultrasound technology offers a clear view of the implant and allows Dr. Mills to evaluate the implant for ruptures and leaks.
What Is High-Definition Ultrasound?
Dr. Mills has been using high-definition ultrasound in his practice for the last 10 years and is very adept with the technology in its application for evaluating the condition of breast implants. Ultrasound is a key component for re-examining breast implant ruptures prior to surgery.
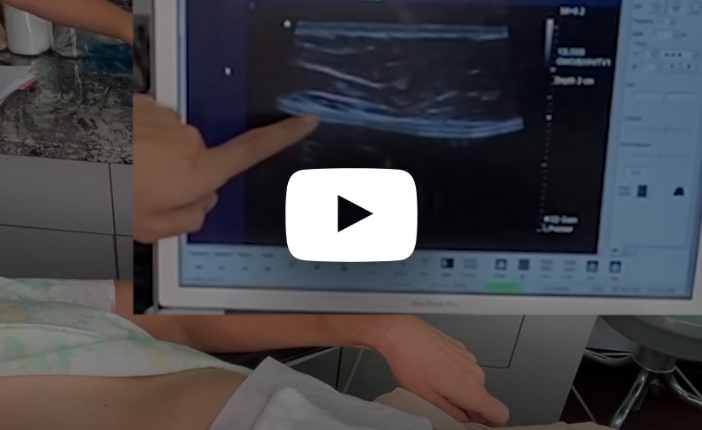
Watch this video of Dr. Mills identifying a ruptured implant on a patient using ultrasound.
Below, you’ll see high-definition ultrasound images that give you a visual idea of how a rupture can be identified. An implant that has scar tissue surrounding it (capsule) should exhibit three lines right together. If you see dark space between the lines, it is likely free-floating silicone around the implant. The silicone surrounds the implant within the capsule. Below, you will see a left side total rupture and a right side intact implant.
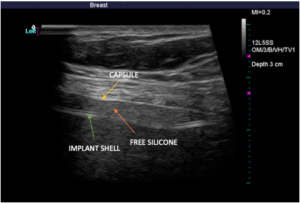
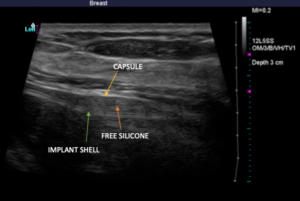
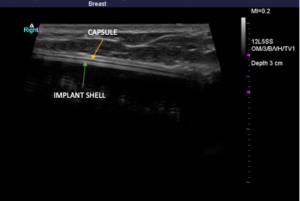
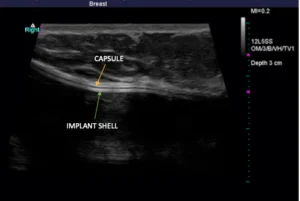
Again, there is no cost to perform high-definition ultrasound imaging for any patient who had their implants placed by Dr. Mills. For individuals who are not breast augmentation patients of Dr. Mills but would like to come in for an ultrasound, he is more than happy to help for an associated fee.
FDA Guidance on Breast Implant Labeling
In 2020, the U.S. Food and Drug Administration issued new guidance with recommendations for breast implant manufacturers. The FDA has recommended that boxed warning labels and a patient decision checklist be included in breast implant labeling to increase the chances that individuals considering breast augmentation are both receiving and understanding important information related to their breast implants. According to the FDA, the boxed warning labels should generally advise patients that the implants are not considered to be lifetime devices, that the risk of complications increases over time, and that some complications will necessitate another surgical procedure. It is also suggested that the warning labels include mention of the fact that breast implants, particularly certain types of implants, have been associated with an immune system cancer called Breast Implant-Associated Anaplastic Large Cell Lymphoma, or BIA-ALCL (which is a very rare condition), and that breast implants have also been associated with systemic conditions (Breast Implant Illness, or BII).
The new FDA guidance also includes recommendations for updating labeling information regarding the recommended protocols on screenings for silicone breast implant ruptures, as well as for incorporation of an easily accessible description of the materials that compose the implant. In addition, the guidance recommends the provision of patient device cards displaying important details about the specific breast implants that have been placed. The FDA is quick to note that these new recommendations for breast implant manufacturers are designed to enhance conversations with the patient’s plastic surgeon, not replace them. Dr. Mills will discuss the risks and benefits of breast implants with you at the initial consultation and he welcomes any and all questions you may have.
Please contact our office if you have additional questions, or if you would like to schedule an appointment.